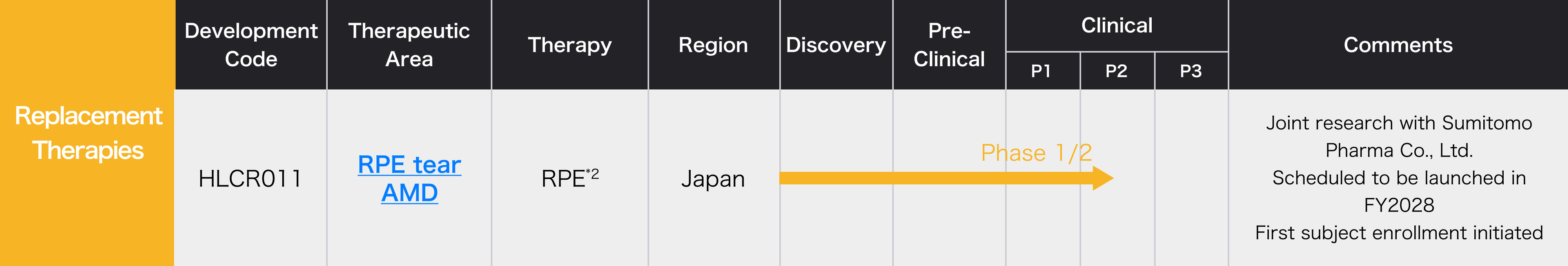
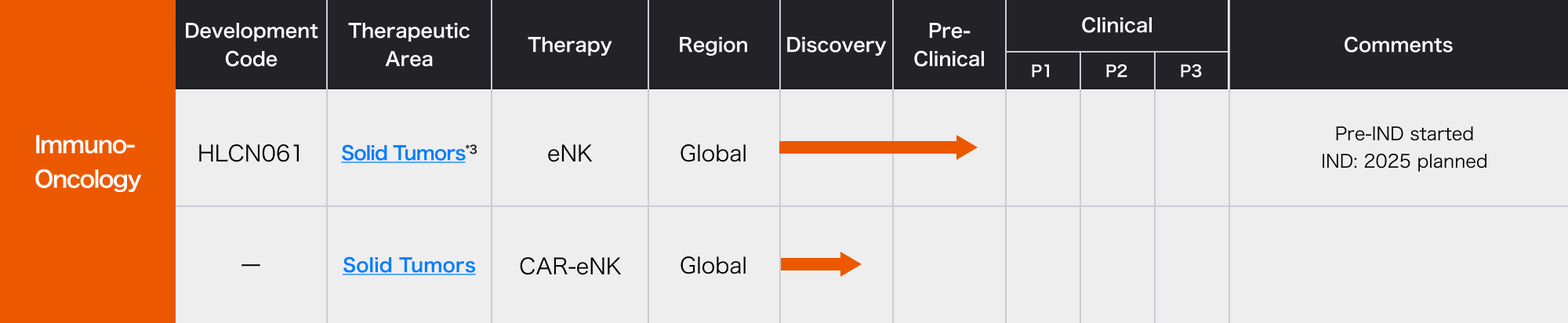
Research and Development Status
- *1 Fast Track and RMAT designations relate to a system that allows for expedited approval of drugs (RMAT is for cellular processed products) that meet certain conditions for the development of new drugs for serious or life-threatening diseases or diseases for which no treatment is available.
- *2 Retinal Pigment Epithelium
- *3 Mesothelioma, Lung cancer, Hepatocellular carcinoma and Gastric cancer,