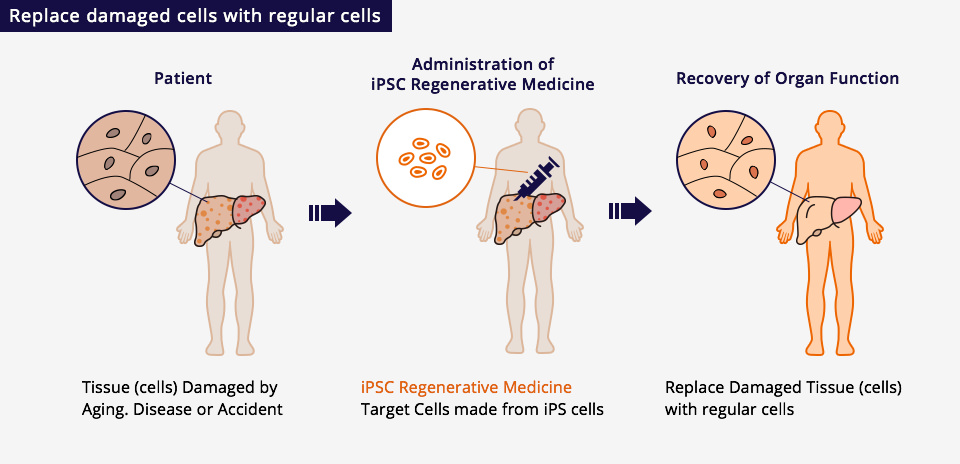
About iPSC Regenerative Medicine
Induced pluripotent stem cells (iPSCs) are established by introducing several factors to somatic cells such as skin cells. iPSCs can differentiate into the cells of a various tissues or organs (pluripotency) and grow indefinitely (proliferation). The objective of iPSC regenerative medicine is to restore cellular function by replacing dysfunctional tissues with cell therapy medicines, which are prepared via a differentiation process (a technique used to artificially change cells into those having specific functions),
and which have the same functions as healthy human tissues.
Research Activities for the Next Generation
Healios is actively engaged not only in alliances with research institutes and companies around the world, but also in its own research activities, in order to quickly establish new technologies and know-how that can become the platform technologies of iPSC regenerative medicine products and accelerate their practical application.
Based on this strategy, we are advancing research activities on next-generation iPS cells, which use gene editing technology to reduce the risk of immune rejection regardless of HLA type. By combining these technologies, we are also making efforts toward the production of next-generation immuno-oncology cells.
