- About RPE tear / AMD
- Current Treatments
- iPSC Derived RPE Cells, The Treatment Healios Is Pursuing
- Development and Production System
About RPE (Retinal pigment epithelium) tear
RPE (Retinal pigment epithelium) tear is a disease in which the sensory retina is detached from the RPE due to a tear in the retina (retinal tear). It causes visual field defects and vision loss. We estimate that about 3,000 people are affected annually (by RACTHERA Co., Ltd. estimation).
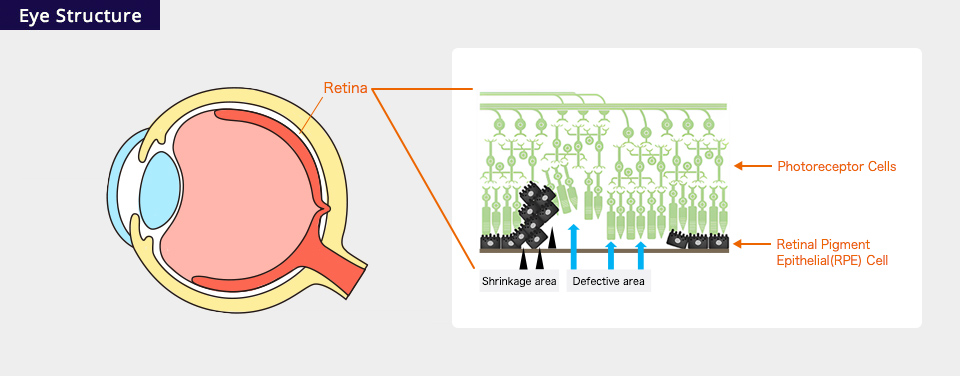
About Age-Related Macular Degeneration (AMD)
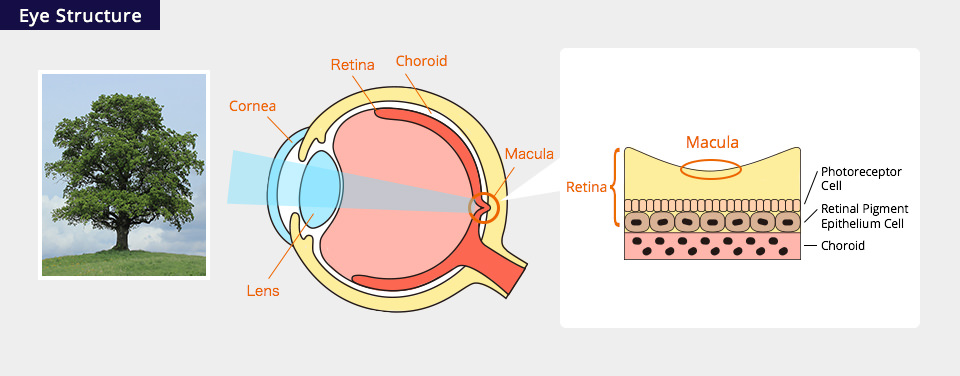
The retina is a light-sensitive nerve layer (similar in function to the film in a camera), lining the inner surface of the human eye. In the retina, light signals are converted to electrical signals and transmitted to the brain via the optic nerve. Eventually, light signals are processed as vision in the visual cortex. In the center of the posterior pole of the human retina, there is a special region known as the “macula.” In the center of the macula is the fovea, 1.5 to 2 mm in diameter, where light entering through the lens is focused, which functions most importantly for visual acuity.
Therefore, if macular function is impaired, sufficient visual acuity cannot be obtained even when other regions of the retina are healthy.
When an older person experiences vision problems because of an impaired macula due to aging, it is referred to as age-related macular degeneration (AMD).
Causes and Symptoms of AMD
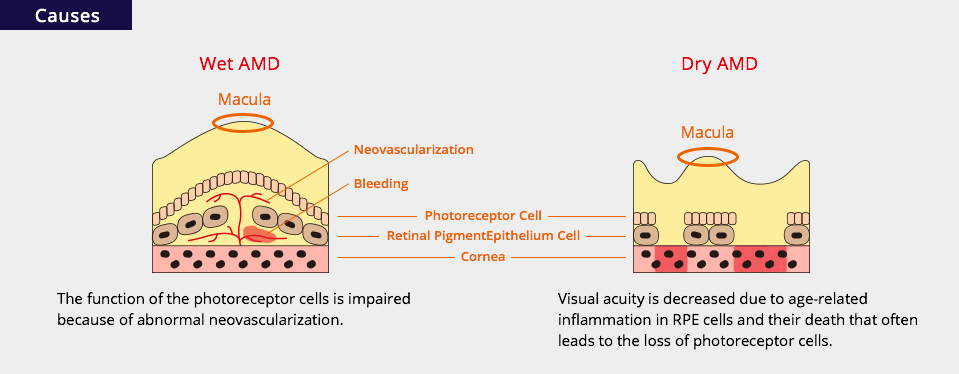
There are two types of AMD, wet (neovascular or exudative) and dry (atrophic), with different etiologies but similar symptoms. Two kinds of tissues, retinal pigment epithelium and choroid, are closely related with the pathology of AMD. The retinal pigment epithelium (RPE) cells form a monolayer, which is localized between the neurosensory retina and the choroid to maintain homeostasis of the retina, especially the macula. The choroid, a well-vascularized tissue, is localized under the RPE cells and nourishes the RPE and retina so they function properly and maintain good visual acuity at the macula.
In wet AMD, the function of the photoreceptor cells is impaired because of abnormal neovascularization, which occurs from the choroid and spreads out below the RPE cells or between the RPE cells and the photoreceptor cells. The neovascularization is caused by RPE cell damage from various causes associated with aging. These new vessels ultimately lead to bleeding or blood component leakage.
In dry AMD, visual acuity is decreased due to age-related inflammation in RPE cells and their death that often leads to the loss of photoreceptor cells.
Number of AMD patients
AMD is the leading cause of vision loss in the elderly in Western countries and the fourth leading cause of vision loss in Japan.
The number of patients with AMD has increased due to longer lifespans of the elderly and a westernized lifestyle, whereas the incidence of AMD was relatively low in Japan until recently. Currently, the prevalence of AMD in Japan is estimated at approximately 1 percent of the population aged 50 years or older and increases with age (Hisayama Town Study, 1998 and 2007).
Current Treatments
Currently no treatment for RPE tear has been established. If RPE cells are missing but photoreceptor function is preserved, RPE cell transplantation can be expected to maintain or restore visual function.
Treatments such as the administration of VEGF inhibitors and photodynamic therapy (PDT) are used to inhibit neovascularization in patients suffering from wet (exudative) age-related macular degeneration. Although both treatments can maintain or improve vision, neither resolves the underlying disease. Furthermore, no effective treatments yet exist for dry (atrophic) age-related macular degeneration.
iPSC Derived RPE Cells, The Treatment Healios Is Pursuing
At Healios, we are pursuing the development of a novel treatment in which new RPE cells, prepared from iPS cells, are transplanted to the affected area since it is suggested that decline in function of RPE cells may cause RPE tear and both wet and dry AMD.
It is suggested that single treatment with the injection of cell suspension involving iPSC-derived RPE cells (liquid involving individually separated cells) or the transplantation of cell sheet consisting of iPS cell-derived RPE cells would be efficient, whereas the current standard treatment for wet AMD requires multiple administration to maintain vision.
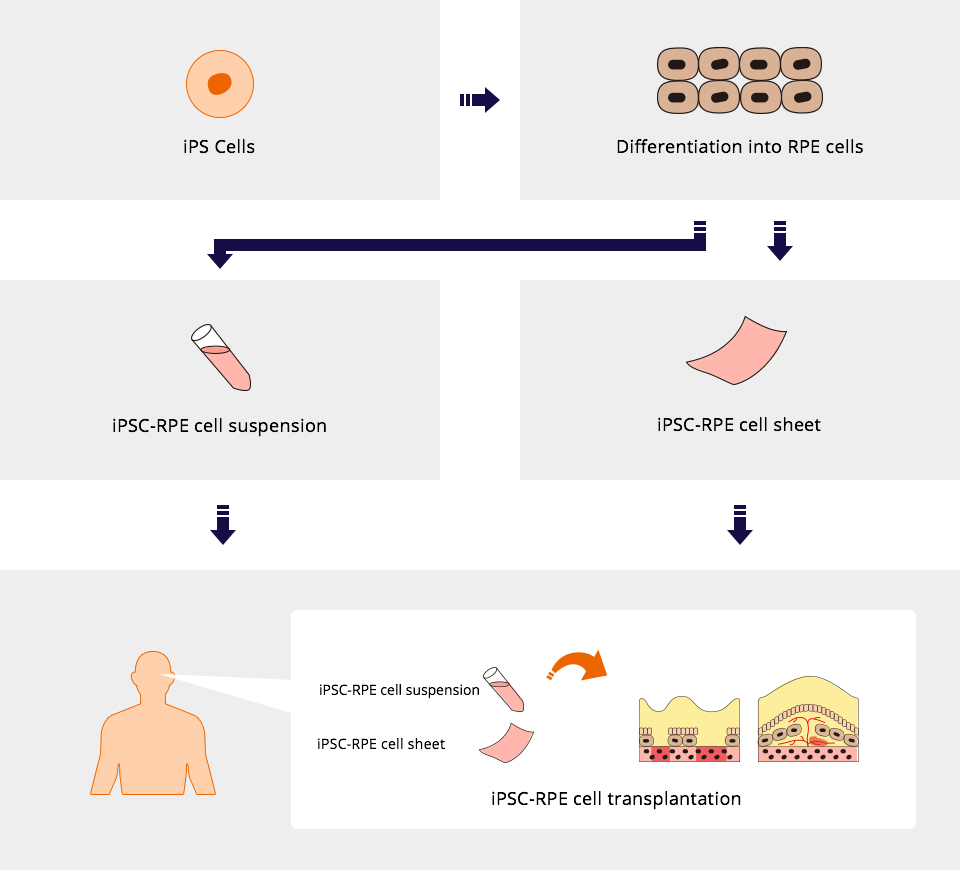
Development and Production System
Healios has obtained the patent license from RIKEN and holds the exclusive worldwide license covering regenerative medicine products that contain RPE cells derived from pluripotent stem cells, including iPSC, as an active ingredient.
Domestically, we have also entered into a contract with RACTHERA Co., Ltd. for the joint development of treatments for RPE tear and AMD using iPS cell-derived RPE cells.
Based on development capital provided by RACTHERA, we intend to jointly develop treatments, for which Healios will obtain approval for production and sales. For joint production and sales promotion of pharmaceutical products involving RPE cells created through these joint development efforts, we have established Sighregen, a joint venture with RACTHERA.
